2.1.1. Eastwood Bio-Medical Research Inc.
Eastwood Bio-Medical Research Inc. (Eastwood) is a privately owned Canadian company engaged in the development and commercialization of a safe and effective treatment for non-insulin dependent diabetes mellitus (NIDDM).
Eastwood’s core technology, P-700 was originally discovered by one of the most highly respected scientists involved in diabetes research, and experts at various universities such as Yale University, University of Calgary and National Institute of Health. They have invested more than ten years screening various plant extracts to discover a suitable natural composition that provides a safe long-term solution for diabetes. P-700 was discovered following numerous experiments and modifications.
Using that technology, Eastwood developed ELEOTIN, a complex proprietary blend of herbs that helps general health promotion, as a dietary supplement.
2.1.2. ELEOTIN
Several years ago, as an unintended by-product from other research projects whose main targets were to find a clue to the anti-ageing substance from natural sources, an initial herbal combination that was effective in regulating blood glucose levels in diabetic animals was discovered. However, the control of blood glucose levels by this first herbal compound was not sufficient to normalize it, even though blood glucose levels after treatment with this first composition are statistically lower than that of untreated groups. Also, it was not determined whether this first composition had any lasting effects on blood glucose levels following the termination of the treatment. The original findings showed an impressive hypoglycemic compound. However, in order to use the substance for a long-term treatment of diabetes, Eastwood had to make numerous improvements and modifications upon this original combination.
The improvements and modifications resulted from the following work:
1) Literature search which produced candidate plants and plant parts,
2) Screening the candidate plants found in 1) through animal tests to find plants with tangible hypoglycemic efficacy and reliable non-toxicity,
3) A second screening of the candidate plants of step 2) with further animal tests to find a narrower list of plants which helped establish possible modes of actions for their hypoglycemic effects,
4) Trying different combinations of the plants which withstood the above screenings,
5) The compassionate human trials, and collection of testimonies,
6) And finally, small-scale systematic clinical trial.
What should be emphasized here as a noteworthy characteristic of the modification process is that all the above steps took place in an interactive fashion, and more than 700 different combinations of different plants have been tested so far.
The followings are some of the herbs contained in ELEOTIN: Platycodi Radix, Schizandrae Fructus, Capsella Bursa, Astragalus Membranaceus Bunge, Nycium Chinese, Dioscorea Japonica Thunberg, Acanthopanax Sessiliflorum SEEMAN.
2.2. Summary of Experiment Results
In what follows, we provide some summaries of various test results in which a current version of ELEOTIN was used. At first, we will review the results of human testing and later animal testing.
Human Test Results
2.2.1. ELEOTIN Controls Blood Glucose Level in Diabetes Patients
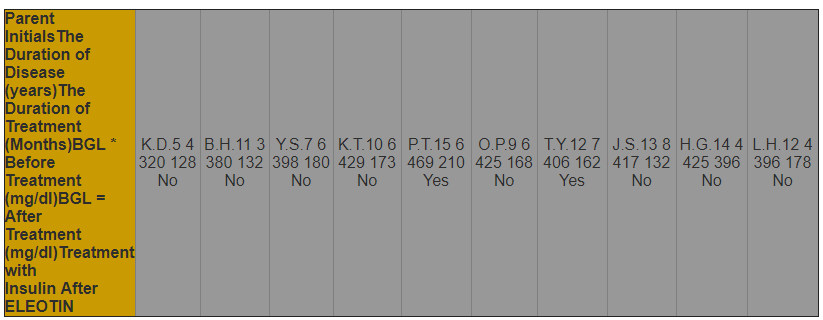
ELEOTIN was administered to diabetes patients. The duration of treatment varied from 3 to 8 months. One result of these experiments is presented in Table 1.
Table 1. Effect of ELEOTIN on the Control of Blood Glucose in Diabetes Patients
| * BGL = Blood Glucose Level |
In various follow-up tests performed in later days, the results are all consistent with the results reported above. However, great variation exists in blood glucose levels among these patients. For example, some patients (~30%) exhibited close to normal blood glucose levels (non-fasting blood glucose level of 120-140 mg/dl). Other patients (~40%) exhibited slightly higher than normal blood glucose levels (non-fasting blood glucose level of 146-178 mg/dl). The remaining patients (~30%) exhibited slightly higher blood glucose levels than all of the other patients (non-fasting blood glucose level of 187-240 mg/dl).
Diet, exercise, and stress, as well as genetic, environmental, and psychological factors, may offer possible explanations for the variation. Differences in the genetic makeup among these patients result in different physiological conditions at the cellular and molecular llevels such as different rates of insulin/receptor up-regulation and different levels of insulin secretion, insulin synthesis, and GLUT2 protein synthesis. Environmental factors, diet, exercise, and stress may be different among diabetes patients. The psychological make-up of the patient (e.g. an individual’s ability to deal with stress) is another important factor when examining the varied responses of diabetes patients to ELEOTIN.
It is noteworthy that some of the patients still have to rely on insulin treatment. Another interesting finding is that even after a few months of the ELEOTIN treatment and in spite of a remarkable reduction of blood glucose levels in all the patients, the blood glucose level of any patient did not go below 128 mg/dl.
Other findings from Eleotin’s Human test results show:
1) Alcohol consumption of any amount reduces the effect of ELEOTIN substantially. In one case, a patient who had suffered serious diabetes for a long time began to show remarkable improvement in all the symptoms related to diabetes after a month of ELEOTIN administration. His blood glucose level approached the normal level, and he was experiencing the improvement of general health.
However, as he resumed the alcoholic consumption from the end of the second month of ELEOTIN use, his blood glucose level reverted to where it was. Subsequently, the patient dropped out of the trial.
In another case, a young patient whose diabetes was recently onset did not stop the alcoholic consumption when he started ELEOTIN usage. The patient did not experience any improvement in the control of blood glucose level within two months. Subsequently, the patient dropped out of the trial. So far Eastwood, at the present time, does not have any systematic studies on the effect of alcohol on the workings of ELEOTIN. The above two cases can be explained by many other factors. Also, the patients could have enjoyed the beneficial effects of ELEOTIN even with their alcohol consumption if they had just stayed a longer period. We report that there were cases where alcohol consumption is negatively correlated with the beneficial effects of ELEOTIN. In any event, consumers taking ELEOTIN should try to minimize their alcohol intake.
2) The age of the patient and the duration of the disease are very important factors that determine the speed of the reduction of blood glucose levels in response to the administration of ELEOTIN. Generally, middle-aged, recent onset cases (6 months to 1year) responded in a few weeks (reaching normal blood glucose levels in a matter of a couple of months) while older patients (over 70) with long histories of diabetes responded as slowly as a year and their blood glucose levels approached the normal levels within two years.
Regardless of the variation among diabetes patients in blood glucose level responses to ELEOTIN, the patients indicated that there was a substantial improvement in many of the general symptoms associated with diabetes. The improvements mentioned by these patients are as follows:
1) An increased amount of urine and reduced frequency of urination resulted.
2) Kidney discomfort, as a result of poor kidney function, is improved, resulting in greater comfort.
3) Interrupted sleep becomes sound.
4) Almost complete relief of pain and stiffness in joints took place.
5) General weakness and fatigue are replaced with an energetic physical condition.
6) Rough skin becomes smooth and soft.
In relation to the improvement of general health, it is noteworthy to mention that the flavonoids and vitamins in ELEOTIN seem to enhance the T cell levels boosting the immune system remarkably, thus helping the users better fight the infections frequently inflicted on diabetic patients.
Interestingly, all the patients felt the improvement of general health conditions before the blood glucose levels actually fell substantially. We interpret this as a consequence of Eleotin’s combinatorial approach, which involves multiple modes of actions which will be explained in later sections. However, we also note a danger that patients who are not monitoring their diabetes closely may have an unjustified sense of “having gotten well”, too prematurely, resulting in premature discontinuance, or negligence of necessary diabetic treatment. In any way, the testimonies of general health promotion are prevalent and consistent. We find it psychologically invaluable considering the chronic nature of the disease in which patients often get depressed and feel helpless.
One interesting observation is that even though most patients experience substantial lowering of the blood glucose levels, they seldom experience full normalization. In other words, it is likely that after a few months of ELEOTIN treatment, the blood glucose level is going to stay slightly higher than the normal level. In contrast, the animals’ blood glucose levels were fully normalized. This difference suggests that Eastwood may have to increase the administered dosage.
Another possible interpretation is that the marginal curative effects of ELEOTIN diminish increasingly as the blood glucose levels approach the normal level, because ELEOTIN relies on ‘mild organic signals’ to the body rather than conveying strong chemical signals for its effect. Mild chemical signals conveyed to the body are likely to be responded more receptively, creating a larger marginal curative effect, when the body is in a more serious condition of diabetes. The body may choose to ignore mild curative signals when the body is not in serious condition. The body may find it better off to stay in a blood glucose level slightly higher than to expensing a large effort to push the level to the normal level.
2.2.2. ELEOTIN Induces Blood Glucose Level Control Stability in Diabetic Patients
Three months after termination of treatment with ELEOTIN, the blood glucose levels of six out of seven patients did not change dramatically (Table 2). The blood glucose levels of one out of seven patients increased slightly (168mg/dl to 186mg/dl).
Table 2. Blood Glucose Levels of Diabetes Patients after Termination of the ELEOTIN Treatment
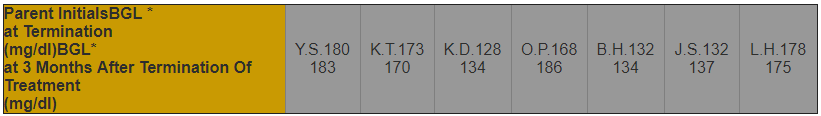
| * BLG = Blood Glucose Level |
We find this result especially encouraging. We see some potential of ELEOTIN as a long-term treatment for diabetes, as well as a temporary treatment. Eastwood hypothesizes that the stable normalization of the blood glucose levels after the discontinuation of ELEOTIN treatment relates to the upregulation of insulin receptors and the regeneration of the ß-cells. Eastwood’s beliefs are also supported by the consistent results from the animal tests that are reported later in this report.
ANIMAL TEST RESULTS
In order to test the effect of ELEOTIN on diabetes and to ascertain the potential modes of actions of such effects, GK rats were used. The GK rat is considered to be one of the best animal models for diabetes. This model is characterized by impaired-glucose-insulin secretion and peripheral insulin resistance. Insulin secretion stimulated by glucose is markedly impaired in GK rats. Insulin response and sensitivity of glycogen synthesis, lipogenesis and DNA synthesis in hepatocytes from GK rats are markedly reduced as compared to non-diabetic control rats such as Wistar-Furth (WF). In GK rats, the islet structure was disrupted and areas of pronounced fibrosis were observed in the stroma. As the disease progressed, ß-cell degranulation was observed, but lymphocytic infiltration of the islet was not. Diabetic complications, such as neuropathy and nephropathy, were observed in biochemically and morphologically.
Treatment of animals with ELEOTIN? results in lower levels of blood glucose and following termination of treatment these blood glucose levels remain low. A period of time effective in treating diabetes may be defined as the length of time of treatment that is required to reduce, and possibly stabilize blood glucose levels. Such a period of time may vary from about 1 to 20 months, more preferably this time could be from about 3 to 10 months.
2.2.3. ELEOTIN Reverses and Prevents Diabetes in Animals
The treatment of diabetic animals with ELEOTIN resulted in a significant decrease in the level of blood glucose and the incidence of diabetes when compared to PBS-treated animals (Table 3, Figure 3A, 3B).
Table 3. Effect of ELEOTIN on GK Rats

| *BGL = Bloog Glucose Level |
| Mean non-fasting blood glucose levels of normal (non-diabetic) Wistar Furth rats, aged 3 months to 7 months, is 145+-17 mg/dl. Any GK rats with a blood glucose level of 230 mg/dl, 5 SD above the mean, were considered diabetic. Rats were treated at 12 weeks of age. Each group contains 20 animals. |
It is apparent that ELEOTIN improves the control of blood glucose, resulting in the treatment of diabetes. When diabetic GK rats were treated with ELEOTIN for approximately 4-5 months, the level of blood glucose of 35-50% of GK rats dropped to the normal range when compared to PBS-treated animals. All PBS-untreated animals remained hyperglycaemic. Furthermore, when GK rats were treated with ELEOTIN for approximately 7 months, most of the animals (85%) exhibited normal glycemia, while all untreated animals remained hyperglycaemic. The blood glucose level of ELEOTIN-treated GK rats was lowered to 146 mg/dl (Table 3).
Normalization and Stabilization of Blood Glucose Levels
Following treatment of GK rats with ELEOTIN for 7 months (Table 4), treatment was terminated and blood glucose levels were monitored for a further 3 months. As Table 4 indicates, after treatment was terminated, symptoms did not return for the duration of the study (3 months).
Table 4. Effect of ELEOTIN on the Control of Blood Glucose Levels of GK Rats after Termination of Treatment

Prevention of Diabetes
Table 5 provides data concerning the prevention of diabetes in GK rats. GK rats usually develop diabetes at 6 to 8 weeks of age. In this experiment, treatment of the GK rats with ELEOTIN (as indicated above) began at 3 weeks of age: before the onset of diabetes. As their blood glucose levels indicate, these animals did not develop diabetes and their blood glucose levels remain in the normal blood glucose range for the duration of the study. In the same study, PBS-treated GK rats exhibited very high blood glucose levels and developed diabetes. Thus ELEOTIN prevented the onset of diabetes.
Table 5 Effect of ELEOTIN on the Prevention of Diabetes in GK rates

| *BGL = Blood Glucose Level |
On the basis of blood glucose levels, ELEOTIN prevents diabetes in GK rats when compared to PBS-treated GK rats. As Table 5 indicates, the blood glucose levels of ELEOTIN-treated GK rats are well within the normal range.
In summary, these results demonstrate that ELEOTIN is effective in treating (Table 3), stabilizing (Table 4), and preventing (Table 5) diabetes in animals. The results are consistent with and strongly supportive of the hypotheses of sections 2.2.1, 2.2.2, where similar indications are reported in human cases.
In order to determine the mechanisms involved in the lowering of the blood glucose level in diabetic animals, Eastwood examined insulin receptors present on hepatocytes and skeletal muscles, the secretion of insulin in the pancreatic ß-cells, and the inhibition of alpha-glucohydrolase catalysed enzymatic reactions, which prevents the degradation of complex carbohydrates to monosaccharides.
2.2.4. ELEOTIN Upregulates the Insulin Receptors in Animals
Decreased insulin-stimulated glucose uptake is characteristic of insulin resistance. The mechanisms involved in insulin resistance in diabetes is unclear but may involve reduced insulin receptor numbers (secondary to hyperinsulinemia and hyperglycemia), reduced tyrosine kinase activity of the insulin receptor, abnormalities distal to the receptor, and defects in the glucose transport system. Glucose transport activity in diabetes is decreased in both adipocytes and muscle.
To determine the effect ELEOTIN has on the expression of insulin receptors, Eastwood measured the rate at which insulin binds to receptors in ELEOTIN-treated GK rats. Briefly, insulin receptors were purified from control and ELEOTIN-treated GK rats using wheat germ agglutinin (WGA) agarose (Klein et al 1986; Burant et al 1986; Vankatesan et al 1991). The rate at which insulin binds to solubilized receptors was determined using 125I-labelled insulin.
The rate at which insulin binds to partially purified insulin receptors from hepatocytes and muscles significantly increased in the ELEOTIN-treated group when compared to the PBS-treated control group (Tables 6A, 6B, Figures 6A, 6B). This difference is strongly suggestive of the up-regulation of insulin receptors in the presence of ELEOTIN. The rate at which insulin binds to the receptors from hepatocytes is greater than the rate at which insulin binds to skeletal muscle receptors.
These results indicate that ELEOTIN may enhance the expression of insulin receptors better in the liver. Eastwood has not yet formed any hypothesis regarding the differentiated upregulation between the receptors from hepatocytes and skeletal muscle receptors. However, Eastwood believes that this is not unrelated to some patients’ testimonies of improved liver conditions after ELEOTIN treatment. Also, there was a case in which a patient with serious sclerosis for whom ELEOTIN did not produce speedy glucose-lowering effects. At this moment, Eastwood believes that the liver plays an important role in the working of ELEOTIN.
Obviously, the effects of ELEOTIN in the upregulation of insulin receptors have significant therapeutic implications:
1) The upregulation of insulin receptors prevents hypoglycemia occasionally caused by excessive insulin secretion stimulated by such hypoglycemic agents as a sulfonylurea.
2) Also, those people who have used insulin injection to control their blood glucose often experience that their needed effective dosage increases over time and the fluctuation of blood glucose levels increases its width. For those people, the upregulation of insulin receptors is significantly helpful to dampen the fluctuation of the blood glucose level and increase the therapeutic effectiveness of the insulin injection.
3) Testimonies of “feeling better”, and ” feeling more energetic” in response to ELEOTIN treatment seems to be a result of this upregulation of insulin receptors.
4) It is shown also that the blood glucose levels of ELEOTIN treated patients stayed normalized after the termination of ELEOTIN treatment. Eastwood attributes this post-treatment normalization to the upregulation of insulin receptors, as well as other causes.
TABLE 6A, FIGURE 6A
Show the rate of attachment of insulin to receptors obtained from the partially purified liver of PBS-treated GK rats and ELEOTIN extract-treated GK rats. Data represents the mean of 10 animals/group.
TABLE 6A

TABLE 6B
Show the rate at which insulin binds to receptors from partially purified skeletal muscle of the hind limb of PBS-treated GK rats and ELEOTIN extract-treated GK rats. Data represents the mean of 10 animals/group.

2.2.5. ELEOTIN Increases the Secretion of Insulin in Animals
In addition to the measurement of insulin receptors of hepatocytes and skeletal muscle, the level of insulin secretion in the pancreas was measured.
In lean, normal, non-diabetic persons, glucose levels are maintained by a balance between insulin secretion from pancreatic ß-cells and insulin action in the splanchnic (liver and gut) and peripheral (muscle and adipose) tissues. Diabetes develops when this balance is upset and impaired ß-cell function (decrease of insulin secretion), and/or abnormal insulin action (insulin resistance) occurs (Leahy et al 1990; Porte 1991; Reaven 1988). Therefore, the decrease of insulin secretion from the pancreatic ß-cells is one of the mechanisms involved in the development of diabetes. In the future, Eastwood will investigate into how ELEOTIN expresses itself in relation with the insulin secretion. For that purpose, PBS-treated or ELEOTIN-treated GK rats were anesthetized with phenobarbital. The pancreas was isolated and perfused as described in the references below. The perfusate (Krebs-Ringer bicarbonate buffer:118 Mn NaCl, 4 Mn Kcl, 2.5 Mn CaCl2, 1.2 Mn MgSO4, 1.2 Mn KH2PO4 25 Mn NaHCO3, 1.2 g/L bovine serum albumin, and 40 g/L dextran) containing 16 Mn glucose was used. The effluent was collected from the cannula in the portal vein at 2 minutes intervals and stored at -20 C. Insulin secretion was measured and calculated as described in Portha et al (1991) and Giriox et al (1983).
The results, as shown in Table 7 and Figure 7 demonstrate that the secretion of insulin from ELEOTIN-treated GK rats increased when compared to PBS-treated control GK rats. This data indicates that ELEOTIN enhances the secretion of insulin from pancreatic ß-cells as a result of an increase in the synthesis of insulin in the pancreatic ß-cells, and/or an acceleration of insulin secretion.
TABLE 7, FIGURE 7
Show the secretion of insulin in response to glucose from the perfused pancreas of PBS-treated GK rats and ELEOTIN extract-treated GK rats.
TABLE 7

2.2.6. ELEOTIN Inhibits alpha-glucohydrolase Breakdown in Animals
Complex carbohydrates present in the diet must be degraded to monosaccharides by alpha-glucohydrolase before they are absorbed in the gastrointestinal tract. In diabetes patients there is less biologically active insulin that utilizes absorbable glucose. Therefore, if the degradation of complex carbohydrates into monosaccharides is inhibited, the amount of absorbable glucose is significantly less, resulting in the requirement for insulin to decrease.
Analysing the inhibition of the degradation of complex carbohydrates into monosaccharides by alpha-glucohydrolase catalysed enzymatic reactions in ELEOTIN treated animals is an essential step to determine the helpfulness of ELEOTIN as a hypoglycaemic agent.
Thus, PBS-treated and ELEOTIN-treated WF rats were fasted overnight and heat-hydrolyzed starch (2g/kg) suspended in water (2g/20ml) was intubated. Fifty minutes later, blood samples were collected and blood glucose levels were determined.
Table 8 and Figure 8 represents the blood glucose level of WF rats administered PBS, PBS and starch, or ELEOTIN and starch. WF rats administered PBS exhibited the lowest blood glucose levels. WF rats administered PBS and starch exhibited the highest blood glucose levels. WF rats administered ELEOTIN and starch exhibited blood glucose levels that were lower than the levels found in WF rats administered PBS and starch, but higher than the levels found in WF rats administered PBS.
TABLE 8, FIGURE 8
Show the effect of ELEOTIN extract on blood glucose from oral loads of carbohydrates in normal WF rats.
These results suggest that the degradation of complex carbohydrate is inhibited because the blood glucose levels of WF rats loaded with carbohydrates (starch) and treated with ELEOTIN were lower than WF rats treated with PBS and loaded with carbohydrates. Because both insulin secretion and insulin action are normal in WF rats, the lower blood glucose level of ELEOTIN-treated rats must have resulted from a lower rate of degradation of complex carbohydrates to monosaccharides, resulting in a decrease of absorbable glucose. Since alpha-glucohydrolase is required for the degradation of complex carbohydrates to monosaccharides, this enzyme must be decreased in ELEOTIN-treated WF rats. In other words, the degradation of starch was inhibited in ELEOTIN-treated WF rats, suggesting that intestinal alpha-glucohydrolase significantly decreased in ELEOTIN-treated WF rats when compared to PBS-treated WF rats.
2.2.7. ELEOTIN Increases the Glucose Transporter in Animals
Glucose enters the ß-cells through a membrane-bound facilitated transporter that is designated GLUT2, or the liver ß-cell transporter. It has been proposed that impaired glucose entry into ß-cells causes a loss of glucose-induced insulin secretion in diabetes patients. This hypothesis is based on the observation that every hyperglycaemic rodent model exhibits a marked reduction of GLUT2 protein in their ß-cells.
To determine whether the level of GLUT2 protein in the pancreatic ß-cells increases in ELEOTIN-treated animals, a Western blot was performed with pancreatic islet homogenates from ELEOTIN-treated GK rats and PBS-treated control GK rats. Preliminary experimental data reveal that ELEOTIN-treated GK rats show an increased level of GLUT2 protein in the pancreatic ß-cells when compared to PBS-treated control GK rats. This result suggests that ELEOTIN may improve glucose transport by increasing the level of transporter protein.
2.2.8. Advantages of Combinatorial Approach
The combinatorial approach with a few modes of actions has a few definite advantages, compared with single mode of action approach.
Firstly, the therapeutic burden on a particular mode is relatively light for the same therapeutic result. Thus, the strain of the therapy on a particular organ, such as kidney and liver, can be lightened by choosing this combinatorial approach.
Secondly, there seem to be certain synergistic effects among these different modes of actions when they are set to work simultaneously. Both quantitative and qualitative results suggest that the combinatorial effect of ELEOTIN is definitely superior to the sum of the effects of individual ingredient. In other words, let’s say herb A has a known mode of action I, and herb B has a known mode of action I, too. Roughly speaking, better therapeutic results are observed when a mixture of half dosages of A and B is used than when either of full dosages of A or B is used. Eastwood believes that slightly different chemical structures of complex compounds help the body to lower its resistance to the therapeutically beneficial substance.
However, we present the above result very carefully because in the course of the modifications of previous versions of ELEOTIN Eastwood also found that some combinations can actually synergistically increase the potential toxicity of each component without increasing the beneficial effects of ELEOTIN. In other words, some combinations may work adversely when mixed together. Eastwood has yet to identify a suitable mechanism of interactions which fully explains these evasive observations. At this moment, Eastwood is satisfied with the discovery of a synergistically superior combination. See the chart below.
2.3. Other Trials and Experiments
2.3.1. Toxicity Test of ELEOTIN in Animals
ELEOTIN appears to be safe for long-term use. It is a herbal combination and all of its ingredients have been used for many thousand years in various countries. And they are recorded as safe in various pharmacopoeia and food codes. For example, all the ingredients of ELEOTIN described in section 2.1.2. are items usable as food ingredient according to the food codes of Korea, China, Japan, and most of other south-east Asian countries. Also, the pharmacopia of a few European countries such as the Netherland show most of the ingredients as safe. For US and Canada, all the ingredients are importable, thus usable as drug and food. The usage of the ingredients has been properly reported to and approved by the food and drug-related regulatory bodies. In order to confirm the safety, a toxicity test with animals was performed as below.
WF rats or SJL/J mice were administered the ELEOTIN extract (50 mg/g body weight i.e., 10x’s the regular dose) every day for seven months. Each animal was sacrificed by CO2 asphyxiation. The esophagus, stomach, intestine, lung, heart, kidney, liver, brain and pancreas of each animal was removed. A small piece of each organ was fixed with formalin and stained with hematoxylin and eosin as described elsewhere (Baek, H.S., and Yoon, J.W. (1990). The stained sections were examined under a microscope (Figure 9).
Esophagus, Stomach, and Intestine: The esophagus (Figure 9A), stomach (Figure 9B) and intestine (Figure 9C) showed intact mucosae and lacked inflammatory cell infiltrates or other features of cellular injury or necrosis.
Liver: The liver (Figure 9D) showed well-defined lobules and cords of hepatocytes separated by anastomosing sinuses and central vein, with no mononuclear cell infiltration, necrosis, or intranuclear glycogen infiltration.
Kidney: The kidney (Figure 9E) showed intact glomeruli, tubules, and vessels. Glomerular changes, tubular strophy, interstitial lymphocytic infiltration, and necrosis were not observed.
Lung: A few dispersed macrophages and normal appearing alveoli, bronchioles, bronchi, and vessels were present in the lungs (Figure 9F).
Heart: The endocardium and myocardium of the heart (Figure 9G) were normal.
Brain: Nerve and glial cells of the cerebral cortex (Figure 9H) exhibited no evidence of necrosis, hemorrhage or infarction.
Pancreas: Endocrine and exocrine cells exhibited intact morphology. No necrosis or lymphocytic infiltration was evident.
The data above indicate that even very high doses of ELEOTIN administered over an extended period of time did not have any deleterious effects on these organs.
Shows the histological profile of the following tissues from WF rats: Figure 9A Esophagus; Figure 9B Stomach; Figure 9C Intestine; Figure 9D Liver, Figure 9E Kidney, Figure 9F Lung; Figure 9G Heart; Figure 9H Cerebral Cortex (x250), after 7 months treatment with 10x’s the regular dose of ELEOTIN.
2.3.2 Further Studies: Implications on Type I
ELEOTIN not only stimulates ß-cells to secrete insulin but also it seems to assist ß-cells regenerate themselves.
Sulfonylurea agents, taken by more than half of the diabetic population, also promote insulin secretion. However, they function only when ß-cells synthesize insulin. And it is reported that they often result in an inadequate amount of insulin secretion by putting strain on the cells.
In contrast, ELEOTIN seems to help insulin secretion and regeneration of ß-cells through general health promotion.
Eastwood has tried ELEOTIN on a few type I patients. They also reported some improvement in control of blood glucose level as a result of ELEOTIN treatment. Eastwood hypothetically attributes such improvement to the aforementioned regeneration of ß-cells.
